Your Relationship between color and wavelength absorbed images are available in this site. Relationship between color and wavelength absorbed are a topic that is being searched for and liked by netizens today. You can Find and Download the Relationship between color and wavelength absorbed files here. Find and Download all free photos.
If you’re searching for relationship between color and wavelength absorbed pictures information related to the relationship between color and wavelength absorbed keyword, you have pay a visit to the right blog. Our website always provides you with suggestions for downloading the maximum quality video and image content, please kindly hunt and find more enlightening video content and images that fit your interests.
Relationship Between Color And Wavelength Absorbed. Therefore when the wavelength is changed the solution will either allow more or less of the wave of color through. Each group should present absorbance vs. The remaining light will then assume the complementary color to the wavelengths absorbed. If it absorbs light in the red and yellow region of the spectrum it will have a blue color.
 Hydrogen Absorption Spectrum Spectrum Chemistry Diagram From in.pinterest.com
Hydrogen Absorption Spectrum Spectrum Chemistry Diagram From in.pinterest.com
We need to work out what the relationship is between the energy gap and the wavelength absorbed. Therefore when the wavelength is changed the solution will either allow more or less of the wave of color through. If the material absorbs blue. The absorbance is greater at 425nm the color at 425 nm is indigo So indigo is the colour absorb the most The set of wavelengths absorbed by a pigment is its absorption spectrumThe set of wavelengths that a pigment doesnt absorb are reflected and t. Hence this is another difference between carotene and xanthophyll. Martin Beckett Martin Beckett.
Compare the data for the mixture with the data for the single solutions.
Each wavelength has a different color that it transmits. Food webs show energy transfer. Βeta-carotene which is a carotene absorbs 450 nm wavelength while lutein and vioxanthan which are xanthophylls absorb 435 nm. Chlorophyll B is the accessory pigment that collects sunlight and passes into chlorophyll A. Q 2What relationship appears to exist between the spectrum for the mixture and the spectra for the individual ion solutions. Q 1What relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution.
 Source: pinterest.com
Source: pinterest.com
The set of wavelengths that a pigment doesnt absorb are reflected and the reflected light is what we see as color. Therefore when the wavelength is changed the solution will either allow more or less of the wave of color through. For part II pooled data are needed. This range of wavelengths. Colored chemicals absorb andor emit light in the visible portion of the electromagnetic spectrum which has a wavelength of approximately 400 700 nm.
 Source: pinterest.com
Source: pinterest.com
However some wavelengths within that range are more effective for excitation than other wavelengths. Many objects contain atoms capable of either selectively absorbing reflecting or transmitting one or more frequencies of light. There is a relationship between an objects color and its temperature at least for black objects and there is a relationship between the wavelength color of a photon and its energy. Follow answered May 16 12 at 1812. If the light absorbed by something is visible this absorption results in the COMPLIMENT of that color being seen by the human eye.
 Source: in.pinterest.com
Source: in.pinterest.com
This relationship is demonstrated by the color wheel shown on the right. The observed colour of complex is the colour generated from the wavelength left over. The set of wavelengths absorbed by a pigment is its absorption spectrum. Compare the data for the mixture with the data for the single solutions. Each group should present absorbance vs.
 Source: pinterest.com
Source: pinterest.com
Chlorophyll a chlorophyll b and β-carotene. The object is to prepare students for part II where the relation between what light is absorbed and what is transmitted will be explored. Food webs show energy transfer. It is easier to start with the relationship between the frequency of light absorbed and its energy. The remaining light will then assume the complementary color to the wavelengths absorbed.
 Source: hu.pinterest.com
Source: hu.pinterest.com
The set of wavelengths absorbed by a pigment is its absorption spectrum. Wavelength and energy are negatively correlated. Food chains only contain animals. The relationship between wavelength frequency and energy means that. The electromagnetic spectrum is used in the lab to demonstrate how the change in wavelength will affect the amount of absorbency and transmittance.
 Source: pinterest.com
Source: pinterest.com
Colored chemicals absorb andor emit light in the visible portion of the electromagnetic spectrum which has a wavelength of approximately 400 700 nm. Therefore there is always a shift along the spectrum between the color of the light absorbed by the fluorophore during excitation and the color emitted. The electromagnetic spectrum is used in the lab to demonstrate how the change in wavelength will affect the amount of absorbency and transmittance. The relationship between wavelength frequency and energy means that. Q 2What relationship appears to exist between the spectrum for the mixture and the spectra for the individual ion solutions.
 Source: pinterest.com
Source: pinterest.com
Martin Beckett Martin Beckett. Food webs are a complex patterns of energy flow. View the full answer. Correct answer to the question What is the relationship between color and wavelength absorbed. Thus absorption of 420-430 nm light.
 Source: pinterest.com
Source: pinterest.com
Higher the crystal field splitting lower will be the wavelength absorbed by the complex. You may want to graph the data for the three solutions. View the full answer. When white light passes through or is reflected by a colored substance a characteristic portion of the mixed wavelengths is absorbed. Colored chemicals absorb andor emit light in the visible portion of the electromagnetic spectrum which has a wavelength of approximately 400 700 nm.
 Source: pinterest.com
Source: pinterest.com
Q 2What relationship appears to exist between the spectrum for the mixture and the spectra for the individual ion solutions. If wavelengths of light from a certain region of the spectrum are absorbed by a material then the materials will appear to be the complementary color Thus for instance if violet light with wavelength of 400nm is absorbed the material will look yellow. The remaining light will then assume the complementary color to the wavelengths absorbed. The absorbance is greater at 425nm the color at 425 nm is indigo So indigo is the colour absorb the most The set of wavelengths absorbed by a pigment is its absorption spectrumThe set of wavelengths that a pigment doesnt absorb are reflected and t. Thus absorption of 420-430 nm light.
 Source: pinterest.com
Source: pinterest.com
Compare the data for the mixture with the data for the single solutions. If the material absorbs blue. Correct answer to the question What is the relationship between color and wavelength absorbed. Therefore when the wavelength is changed the solution will either allow more or less of the wave of color through. Wavelength and energy are negatively correlated.
 Source: pinterest.com
Source: pinterest.com
Q 2What relationship appears to exist between the spectrum for the mixture and the spectra for the individual ion solutions. Correct answer to the question What is the relationship between color and wavelength absorbed. Therefore when the wavelength is changed the solution will either allow more or less of the wave of color through. Looking at the spectrochemical series H 2 O is a weak field ligand so it absorbs colors of long wavelengthsin this case the longer wavelength is yellow so the color reflected is violet. It is easier to start with the relationship between the frequency of light absorbed and its energy.
 Source: pinterest.com
Source: pinterest.com
Red is the lowest energy visible light and violet is the highest. The set of wavelengths that a pigment doesnt absorb are reflected and the reflected light is what we see as color. Food webs are a complex patterns of energy flow. Looking at the spectrochemical series H 2 O is a weak field ligand so it absorbs colors of long wavelengthsin this case the longer wavelength is yellow so the color reflected is violet. Many objects contain atoms capable of either selectively absorbing reflecting or transmitting one or more frequencies of light.
 Source: pinterest.com
Source: pinterest.com
The colors perceived of objects are the results of interactions between the various frequencies of visible light waves and the atoms of the materials that objects are made of. Follow answered May 16 12 at 1812. By Radhe Gupta October 4 2021. Compare the data for the mixture with the data for the single solutions. Wavelength and energy are negatively correlated.
 Source: pinterest.com
Source: pinterest.com
Each group should present absorbance vs. You can see that if you want a high energy jump you will have to absorb light of a higher. When white light passes through or is reflected by a colored substance a characteristic portion of the mixed wavelengths is absorbed. A fluorescent dye absorbs light over a range of wavelengthsand every dye has a characteristic excitation range. In the diagram below you can see the absorption spectra of three key pigments in photosynthesis.
 Source: pinterest.com
Source: pinterest.com
Difference Between Chlorophyll A and B Contribution in Photosynthesis. The absorbance is greater at 425nm the color at 425 nm is indigo So indigo is the colour absorb the most The set of wavelengths absorbed by a pigment is its absorption spectrumThe set of wavelengths that a pigment doesnt absorb are reflected and t. Food webs contain both plants and animals. It is easier to start with the relationship between the frequency of light absorbed and its energy. Looking at the spectrochemical series H 2 O is a weak field ligand so it absorbs colors of long wavelengthsin this case the longer wavelength is yellow so the color reflected is violet.
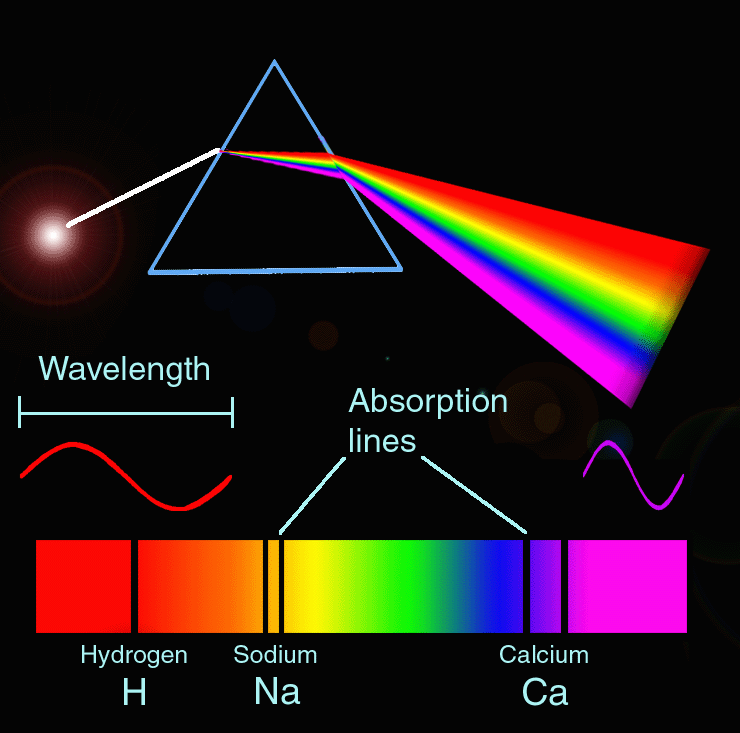 Source: pt.pinterest.com
Source: pt.pinterest.com
When white light passes through or is reflected by a colored substance a characteristic portion of the mixed wavelengths is absorbed. As the wavelength of an electromagnetic wave get shorter and its frequency increases and the amount of energy it transports becomes greater. If wavelengths of light from a certain region of the spectrum are absorbed by a material then the materials will appear to be the complementary color Thus for instance if violet light with wavelength of 400nm is absorbed the material will look yellow. This answer is incorrect. Relationship between color and wavelength absorbed.
 Source: pinterest.com
Source: pinterest.com
Higher the crystal field splitting lower will be the wavelength absorbed by the complex. Food webs show energy transfer. The relationship between wavelength frequency and energy means that. Each group should present absorbance vs. Thus absorption of 420-430 nm light.
 Source: pl.pinterest.com
Source: pl.pinterest.com
Each wavelength has a different color that it transmits. CN-is a strong field ligand so it absorbs colors of shorter wavelengths-in this case the shorter wavelength is violet so the color reflected is yellow. Chlorophyll A absorbs the light in the range of 430 nm to 660 nm. Chlorophyll a chlorophyll b and β-carotene. A solid object has color depending on the light it reflects.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title relationship between color and wavelength absorbed by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






